When you add water to your pool, you also add dissolved calcium (and magnesium) carbonate. Adding hard water means adding a lot calcium carbonate. Water evaporates, the calcium carbonate, dissolved in it, does not. Your pool water gets harder and harder. In time there will be a point where there is simply too much calcium carbonate to keep in solution.
Consequences of excessive hardness can result in scaling damage to the pool, or stated a bit different, a nightmare! Dealing with high levels of calcium hardness in swimming pools can be very difficult. This page is intended to list practical options.
I’m writing this article because I can’t find good guidance for a thorough approach. Help is greatly appreciated, feel free to leave a comment.
Why is this page so important? The main tool to keep you out of danger, preventing scaling, is keeping the pH low. This is the trick often applied in private pools. However a low pH does have serious consequences for swimmers, varying from red eyes to out of control patogens in the water.
Table of Contents
Be prepared
A hard water pool needs a special approach. The following points are important.
- Check hardness more often.
- As mentioned, water evaporates, calcium carbonate does not. When hardness gets critical, you may have to act, even break laws such as going below pH 7.2 and doing the not so logical, like replacing (too) hard water with hard water.
- Learn to recognize signs of over saturation before scaling damage, such as milky water, white calcium carbonate particles (caused by reacting with carbon dioxide gas?) at waterline level.
- Solubility of calcium carbonate is crucial. See also calcium carbonate solubility parameters at Wikipedia. Several parameters are at play and luckily can be tuned. Some quick conclusions can be drawn. To keep solubility of calcium carbonate as high as possible:
- Never use swimming pool chemicals that contain calcium.
- Keep the pH on the low side so that calcium carbonate remains in solution as good as possible. pH 7.2 or slightly above.
- Keep alkalinity on the low side. 80 ppm for example.
- Lower the water temperature. Can you create shade? Or cover the pool?
- Raise salinity to a value that won’t harm your pool. See below for more information.
An overview of solutions
Use tap water
The obvious, but still… If you have soft tap water at your disposal, consider, reconsider using it. You can mix soft and hard water as long as you keep the hardness under control. Using soft water is often the cheapest and best solution.
Apply water softening techniques
Even a (relative) small resin based water softener (example) can handle a pool in days. I really like the idea. There are two scenarios:
- Soften the water that is in the pool. Preferred, no draining. However, high salinity is a showstopper here – more than 1000 ppm. In that case the only option is:
- Soften the water used to fill the pool and drain a part of the water when evaporation causes too much hardness.
An example of an installation:
This one is a equipped with a proper volume (not time) based control valve, 45 dm3 per minute flow, a 35 dm3 tank and 150 kg salt capacity. The beauty is the price, wharehoused on several spots on earth and least maintenance.
You may want to search the net for “CoronWater”, model names like “cws-cst-1044”. If you are experienced with this device, please drop a note below.
Keep solid particles in solution
Consider using a flocculant like PAC or aluminium sulphate to bind solid calcium carbonate particles and filter it out. I can’t find in depth information about this.
Filter with zeolite instead of sand
There is contradicting information on the net and so far I don’t know what to believe. A 100 m3 pool with 1000 ppm calcium carbonate means there is 100 kg calcium carbonate, 70 kg too much. Input more than welcome!
Precipitate calcium carbonate
Tricky and messy but tempting! Use sodium carbonate or sodium hydroxide to undissolve calcium carbonate and use alum to bind particles and let them sink. Finally remove the particle cloud at the bottom of the pool. See this example. This is some sort of last resort solution. However, on the other hand…
Thinking out of the box, literally, it is tempting to do this process not inside, but outside the pool. For example:
- Fill one or more IBC containers with pool water.
- Raise pH to say 10, when the solution gets cloudy, it is a sign of not dissolved calcium.
- Wait for the calcium salts to sink and the water is clear again. You may want to get an idea of how hard the water is.
- Pump the clean water back to the pool and also add hydrochloric acid in the pool in order keep the pH of the pool under control. Repeat until you’re satisfied.
Raise salinity
Salt water can contain more calcium carbonate. I really find it odd that this is never mentioned in pool blogs. Studies confirm the strong relation. For example, search the net for “relation salinity and calcium carbonate solubility”. Subjects are mostly based on ocean water. It is the Wikipedia link that provides swimming pool considerations. The link to the study also considers brackish water, see ![]() values at page 150 for example.
values at page 150 for example.
Some considerations:
- For example, a Zodiac LM3 chlorinator can operate between 4000 and 13000 ppm.
- Bear in mind that some metals don’t like high salt concentrations, be careful at levels above 6000 ppm.
- Also take into consideration that
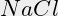 is a by-product of several reactions in your pool. Salinity can rise in time without adding salt, without you noticing it.
is a by-product of several reactions in your pool. Salinity can rise in time without adding salt, without you noticing it.