This article allows you to make disinfectant yourself. Clean hands are one of the keys to not getting infected with COVID-19. What to do if you can’t get disinfectants like water and soap when you are on the way and alcohol based disinfectants are sold out? How do you make hand sanitizers yourself? There are the recipes for disinfectant from the World Health Organization (WHO). They can be seen as acceptable and are used worldwide although the recipe is somewhat older (2009). This post is about the way to make these disinfectants for yourself, and the people around you, as a 1 litre amount. WHO “Formulation 1” is based on ethanol (EtOH) and “Formulation 2” is based on isopropanol (IPA). They are both effective against coronaviruses. You can use them as hand sanitizer and more general.
Warning: incorrect interpretations of this recipe are also in circulation. Therefore, Formulation 1 has been analysed and Formulation 2 has been determined empirically. It’s a long line of reasoning that may be less interesting. I finish with the WHO recipes based on both mass and volume. If you have any additions or questions, feel free to respond. If disinfectants and hand sanitizers are scarce, this is one way to ensure hygiene. Make sure to read the warnings.
There is only one major drawback, alcohols are extremely flammable. Consider that risk and stay alert, with clean hands in a burn centre is the last thing you want. If you want to make disinfectant hand sanitizing yourself: I will not stop you, but read the entire article, take warnings very seriously. In other words, rather buy an existing available sanitizing liquid. Okay?
Obsessively disinfecting your hands all day is a bad idea, sterile hands are themselves prone to other infections. So you also have to think a bit, rather disinfect the handle of the shopping cart beforehand than disinfecting your hands afterwards. Get rid of the habit to touch your mouth, nose and eyes (with dirty hands). Stay away from sniffers and so on. Having said that, after using your hands in public places, you may want your hands disinfected and alcohol based cleaning is often the only thing available.
Table of Contents
Warnings
- Stick to the text of the WHO recipe and read it carefully.
- The substances available may not always be of pharmaceutical quality. You should check this. This might not be a problem for use as a disinfectant, but it is important to realize this. Absorption through the skin is limited during normal use.
- Ethanol metabolites are socially accepted.
- Methanol and isopropanol metabolites are formaldehyde and acetone, respectively, toxic in higher concentrations, occurring naturally in the body and can be excellently metabolised in small quantities.
- To get an impression of the composition of a bioethanol you can consult this pdf.
- Disinfectant, ethanol and isopropanol are extremely flammable and difficult to extinguish. Water will initially backfire. Ensure quick access to CO2 or powder extinguishers and a (fire) blanket. You, and other users, have to be aware of the fire risk, both during production and use.
- Hand pumps and hand sprays: Always use small bottles, 50 ml for example.
- After application, always be careful with lighters, candles, gas stoves, etc., until the mixture has evaporated on your hands. This also means: Always apply near an area with tap water to extinguish – dilute the solution.
- Diluting with plenty of water is an option when it comes to a burning small amount, for example on your hand or a 50 ml container. Unless no alternative is available, don’t extinguish larger quantities with water, this will initially increase the fire. Do use a water spray mode to cool and dilute if possible. Never store larger quantities indoors.
- Reuse the alcohol packaging for storage, which is made for that. Choose a cool, dark, ventilated and safe place out of the reach of children.
Usage
The use is as hand sanitizer is easy, apply it on your hands, keep rubbing until everything has evaporated. Do not rub your hands on a towel, the liquid should be able to act for about 30 seconds. If you want to know what is effective against the COVID-19, or actually the family of coronaviruses, in a broader sense, you should consult this document.
Disinfecting objects: Similar, give it time to do the job. Dirt can be preprocessed be moisturising it first. You may want to use a solution without glycerol for this – glycerol residues themselves are also a pollution.
How does it work against bacteria and viruses?
In chemistry, “alcohol” is slightly different from the liquor store. Alcohols are a family of chemically related substances, including the “ethanol” and “isopropanol” used herein. The alcohol in spirits is “ethanol”.
“More is better” is not always true, pure ethanol is not nearly as effective as ethanol to which water has been added. On the other hand, an ethanol solution of 70% or less is also less effective – so look closely at packaging of commercial products.
The water present is necessary to make cell walls of bacteria permeable to the alcohols. The alcohols eventually destroy the internal cell. A large part of the viruses, coronaviruses like COVID-19, is also sensitive to this disinfectant. More specifically, alcohol dissolves the protective fat layer and denatures – breaks down – the (genetic) proteins.
General considerations
- Tap water is often available in toilets and bathrooms, which makes it possible to use it there. Application in kitchens often means applying in the vicinity of various ignition sources. As long as the alcohol has not evaporated, the risks are high and unacceptably high for small children without supervision.
- Finally, there is the discussion about drying out the skin. Alcohols, especially isopropanol, dissolve skin fats. Therefore, wiping your hands is not a good practice. If the alcohols can evaporate, the skin fat will eventually remain behind. In addition, wiping your hands significantly reduces the degree of disinfection.
- The concentration is important for the degree of disinfection.
- Not too much alcohol, not too much water.
- WHO assumes v/v (volume/volume)
- 80% for EtOH disinfectant.
- 75% for IPA disinfectant.
- The literature is not enthusiastic about EtOH <70%. So sticking to those WHO values is what we do.
- WHO uses a margin of error for the alcohol percentage of +/- 5%.
Technical considerations
- Calculating with volumes can be deceptive, if 1 litre of ethanol is mixed with 1 litre of water, then you do not have 2 litres of liquid. Here interpretations of the WHO recipe can go wrong because too little water is added and the final result is a less effective disinfectant. That is a reason to work with mass (weight) and to write this page. A proper kitchen scale comes in handy.
- Sources: https://vanderworp.org/wp-content/uploads/2020/03/vvvsmm_.svg, derived from https://vanderworp.org/wp-content/uploads/2020/03/vvvsmm.dwg_.zip.
- Wherein known measuring points (grey crosses, source lost) are connected with a green NURBS. It is therefore fairly reliable to extract information from the dwg.
- Additionaly, a mathematical curve is defined through the magenta circles as an appropriate polynomial:
- The goal is to find a suitable function that goes through a number of points. This is plotted as the hardly see-able red curve. That function is plotted for checking with a x=1 resolution. Because we are going to work in GNU Octave, the notation is conform:
- There are 5 definition points chosen for a 3rd degree function:
x=[54.1,70,85,95,100];y=[62,77,89.5,96.8,100];n=3;- What function does that provide?
v = polyfit(x,y,n)v = -0.000022796 0.001287985 1.047688655 5.161471442- And thus a notation as function:
(1)
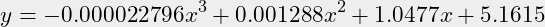
- Densities are given and inaccuracies will have little effect. Water has a maximum density of 1 kg / litre and will be lower at temperatures other than 4 degrees Celsius. Salts present increase the density, so 1 kg / litre is an acceptable assumption. Densities in kg / litre or gram / ml
- Water: 1
- Ethanol: 0.789
- Isopropanol: 0.786
- Glycerol: 1.261
Reverse engineering of the WHO recipe
Or: What was the WHO reasoning?
- There are two WHO recipes to supplement up to 10 litres with sterile distilled or cold boiled water. These are the components:
- As a base EtOH
- EtOH 96%: 8333 ml
- Hydrogen peroxide 3%: 417 ml
- Glycerol 98%: 145 ml
- Top up to 10 liters with water
- As a base IPA
- IPA 99.8%: 7515 ml
- Hydrogen peroxide 3%: 417 ml
- Glycerol 98%: 145 ml
- Top up to 10 liters with water
- As a base EtOH
Approach 1: The IPA solution
Because almost pure IPA is used in recipe 2, that is a starting point. I start easily.
Parts based on v/v: IPA 0.998, Water 0.002. Water part of 7515 ml: 0.002 * 7515 = 15 ml. So there is 7515 – 15 = 7500 ml IPA per 10000 ml. This corresponds exactly to 75% IPA. So WHO doesn’t make things complex, beautiful!
Approach 2: The EtOH solution
Does approach 1 also apply to EtOH? Here is the check, pretending that the volume of the mixture does not shrink.
Parts based on v/v: EtOH 0.96, Water 0.04. Water portion of 8333 ml: 0.04 * 8333 = 333 ml. So there is 8333 – 333 = 8000 ml EtOh per 10000 ml. This corresponds exactly to 80% EtOH.
Conclusion
WHO mentions the percentage of alcohol, meaning to say: “Before it is mixed”. The shrinkage of the mixture is not taken into account. To arrive at 10 liters, more additional liquid will therefore have to be added. That is the basis for the calculations below.
The conversion to mass
I have the shrinkage data for mixtures of ethanol and water, see the graph above, I do not have this for isopropanol and water. So analysis of the recipe with isopropanol is empiric.
Analysis of the EtOH recipe
Let’s start with a 1-liter WHO recipe based on EtOH. That temporarily fits into a PET bottle with a tight-fitting cap, for example. When you’re done, divide it into 50 ml bottles and the rest goes back into an empty alcohol bottle with a new label. Do everything outside and have extinguishers within reach.
- How much is the desired 80% ethanol if we use 95% bioethanol as a base?
- 80% of 1 liter is 800 ml of pure ethanol. A total of 0.789 g/ml * 800 ml = 631 grams of ethanol is therefore required as total amount in 1 litre end product.
- 95% v/v bioethanol is 92.3% m/m. 1 gram of 95% bioethanol contains 0.923 grams of ethanol (and 0.077 grams of water). Therefore, 631 g / 0.923 g/g = 684 g of 95% bioethanol is required.
- Additionally it contains 684 – 631 = 53 grams of water.
- What is the volume of 684 gram of 95% bioethanol?
- 1 litre of 95% bioethanol contains 950 ml of pure ethanol, 950 * 0.789 = 750 grams. The corresponding volume of 631 grams of pure ethanol corresponds to 631/750 = 0.841 litres = 841 ml.
- The easiest ingredient is hydrogen peroxide because we do not add it – only if all substances are free of harmful bacterial spores. So 0 grams. If you want to add the 0.125%: 1.25 ml of 100% hydrogen peroxide with a density of 1.45 g/ml equals 1.81 grams. 41.7 ml of 3% hydrogen peroxide with a density of ~1,014 g/ml equals 42 grams.
- Then there is glycerol. For 10 litres 145 ml is needed, so for 1 litre 14.5 ml. Expressed in grams: 14.5 ml * 1,261 g/ml = 18 grams.
- Finally the water – sterile, at least boiled and cooled down.
- We have assumed 80% v/v, without shrinkage. Based on mass percentages with shrinkage, this corresponds to 73.5% m/m. So if 631 grams of ethanol is 73.5%, then the water part equals: (100 – 73.5) * 631 / 73.5 = 228 grams.
- The used 95% v/v ethanol already contains 53 grams of water, so we should add 228 – 53 = 175 grams or ml of water.
- If glycerol and hydrogen peroxide are used, their respective volumes must be subtracted from that 175 ml: 175 – 15 – 42 = 118 ml or grams of water.
So everything can now be summed up in a recipe.
Determination of the IPA recipe
The WHO isopropanol (IPA) disinfectant recipe is based on volume. Since data on the density of mixtures of water and IPA are lacking, I will start with kitchen tools myself. So there may be a limited margin of error. Then the WHO IPA recipe can be noted. As a final piece, I assembled some data found, in order to be able to offer a reasonable overview of mixtures of water and IPA.
Resume
- The mass and volume of a glass bottle are determined to a mark.
- 75% of this volume is supplied with IPA by taking mass of IPA as a starting base – IPA density in kg / liter or gram / ml: 0.786.
- Glycerol and hydrogen peroxide are not added.
- The volume is supplemented from 75% to 100% with boiled and cooled water. The mass is weighed.
- Everything is summarized into a recipe with both mass data and volume data.
Hands on
- Do everything outside and have extinguishers within reach.
- An old Port bottle, without cork, is cleaned and dried.
- Empty mass: 495 grams
- Right under the top there is a mark. Filled up with water. Total mass: 1260 grams, water mass: 1260 – 495 = 765 grams.
- Total water mass equals bottle volume: 765 ml.
- 75% of (99.9%) IPA is 765 * 0.75 = 573.8 ml.
- Corresponding mass: 573.8 ml * 0.786 g/ml = 451 grams.
- Empty the bottle
- Fill the bottle with 451 grams of IPA, total mass: 946 grams.
- Add water to the mark. Shake to make sure it is mixed well before adding the latest drops.
- Measure the total mass: 1155 grams.
- Label the bottle.
Conclusions with a reasonable tolerance
- Based on the test with the bottle:
- Results come from unverified and fairly inaccurate means. The temperature was ~ 20 degrees Celsius.
- The amount of water added: 1155 – 946 = 209 grams.
- The density of a solution with 75% v/v IPA: (209 + 451) / 765 = 0.863 g/ml
- 75% v/v IPA corresponds to: 100 * 451 / (209 + 451) = 68.3% m/m.
- Converted to one litre of disinfectant:
- IPA: 750 ml or 750 ml * 0.786 g/ml = 590 grams.
- Water: 209 * 1000 / 765 = 273 gram = 273 ml
Additional data
It is extremely disappointing that this type of obvious data is behind the walls of publishers and is not public. Almost endless copyright and data paid by tax payers not being public, is a political choice of lawmakers, serving lobbyists, where a responsibility to protect community interests also lies with the citizen. The only option that remains is to collect data from various sources and combine it. My analysis is prone to relative small errors and concludes with 0.863 g/ml for 75% IPA. It is kept outside the definition points of the function.
- Points collected
- 100% IPA has a density of 0.786 g/ml according to various sources.
- Collected from various sources in links: 70% IPA is 0.88 g/ml, 50% IPA is 0.92 g/ml, 30% IPA is 0.952 g/ml, 20% IPA is 0.98 g/ml.
- 0% IPA has a density of 1.0 g/ml according to various sources.
- There are then 6 points for a third degree function.
y=[1,0.98,0.952,0.92,0.88,0.786];x=[0,20,30,50,70,100];n=3;- Gnu Octave comes up with these parameters:
-0.000000071276 -0.000000487711 -0.001388486586 1.001523213086
- This provides a function for calculating density “d” in g/ml for a percentage “x” between 0% and 100% v/v:
(2)
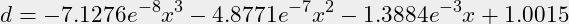
- Using the function for 75% v/v returns 0.865 g/ml, considering that the function is based on only six points where several points only have two decimals precision, it is acceptable to use the empirical retrieved density for 75% v/v IPA of 0.863 g/ml.
- Please consider an error margin of a bit more than 1% when using the function.
The recipes for disinfectants
- Recipes for 1 litre disinfectant.
- Millilitres and grams can be used interchangeably, the end result is the same.
- Unlike the WHO recipes, no glycerol and hydrogen peroxide are added. If you want to add it, replace:
- 15 ml of water for 15 ml or 18 grams of 98% glycerol.
- 42 ml of water for 42 ml or 42 grams of 3% hydrogen peroxide.
Adapted recipe based on 95 percent bio-ethanol
- WHO Formulation 1, without glycerol and hydrogen peroxide
| Substance | Mass (gram) | Mass (%) | Volume (ml) |
| Bio-ethanol, 95% v/v | 684 | 80 | 841 |
| Water, boiled and cooled or sterile and distilled. | 175 | 20 | 175 |
| total | 859 | 100 | 1000 |
Adapted recipe based on 99.9 percent isopropanol
- WHO Formulation 2, without glycerol and hydrogen peroxide
| Substance | Mass (grams) | Mass (%) | Volume (ml) |
| Isopropanol, IPA, 99.9% v/v | 590 | 68 | 750 |
| Water, boiled and cooled or sterile and distilled. | 273 | 32 | 273 |
| total | 863 | 100 | 1000 |
Hello,
I have the same problematic for creating WHO hand desinfectant.
I also use 99.9 IPA alcohol.
I mixed 59.25g (approx 75.08ml) of 99.9 IPA with 24.4ml water (later I will add 0,5ml of glycerol). I obtained a density of approx 0.860. Wich seems to be a little over 74% according to this chart : https://www.aat-corp.com/ipa-water-density-percentage-chart, instead of 75.37% in theory.
I don’t know really where the little error come from, I will probably add a little bit more Ipa to meet the density 0.858
I’m not saying your datas are wrong, I’m just looking for more information.
Can you share your sources for the densities of ipa mixture ?
I’m a bit nervous about making hand sanitizer, because it’s for my mother small shop.
First of all, WHO takes a tolerance of +/- 5% v/v into consideration, so I think it is hard to make serious errors. I hope you understand 75.08 ml + 24.4 ml is not 99.48 ml. The sources I used are from the products sold by industry, there are links to each datasheet where density is given in two decimals. It differs a bit from the table you use: http://www.labchem.com/tools/msds/msds/VT380.pdf says 0.88 g/ml where the above link mentions 0.871 g/ml. I don’t think you should worry too much since it is all inside the tolerance limits.
Can Methanol be used as a disinfectant for Covid 19
It is likely to kill COVID-19. However, breathing in vapors and skin contact will also lead to methanol in your body. The liver turns methanol into formaldehyde, which is a serious health risk, even with smaller levels: blindness, kidney failure and death.
Dear Sir
Thank you very much for guided recipe it helps a lot
I understand it is free to use this recipe
In order to distribute this I would be needing and MSDS
Is there a possibility to receive the related MSDS for the various Ethanol/IPA formulations.
Hans Blom Chemical industry expert
No idea, this page is intended for small scale personal use, no SDS needed 😉
Zou je in plaats van bio ethanol ook niet zelfgestookte ethanol van bijvoorbeeld 75 vol% kunnen gebruiken? Je zou ook sterkere ethanol kunnen aanlengen met water en de sterkte meten met een alcoholmeter (hydrometer) dacht ik?
In theorie wel, de gevaren van methanol-OD zijn kleiner omdat opname door de huid beperkter is dan bij drinken. Je zou teststrips in kunnen zetten om dat risico weg te kunnen nemen. En de andere kant op werken met een hydrometer is altijd een optie. Wel omslachtig allemaal.
If I add glycerol and hydrogen peroxide which is correct: (1) 15 g of water for 18 g of 98% glycerol or (2) 18 g of water for 18 g of 98% glycerol.
(1)1.25 g of water for 1.3 g of 3% hydrogen peroxide or (2) 1.3 g of water for 1.3 g of 3% hydrogen peroxide
Two times answer (1):
EDITED:
“Unlike the WHO recipes, no glycerol and hydrogen peroxide are added. If you want to add it, replace:
15 ml of water for 15 ml or 18 grams of 98% glycerol.
42 ml of water for 42 ml or 42 grams of 3% hydrogen peroxide.”
Why did you use 1.25 ml in the calculations instead of the WHO recommended 417 ml?
That does not feel right and I think you hit a bug. Let’s see, https://www.who.int/gpsc/5may/Guide_to_Local_Production.pdf mentions at page 3 417 ml 3% hydrogen peroxide for 10 litres, 41.7 ml/l. At page 4 the final concentration of hydrogen peroxide is mentioned as 0.125% (v/v), being 1.25 ml. Apparently and also obvious, they were meaning total amount of hydrogen peroxide, not the 3%. If the 3% solution is 41.7 ml, the amount of 100% hydrogen peroxide is 41.7*3/100= 1.25 ml. That is not based on 3% hydrogen peroxide, but on the total (100%). So it should be 41.7 ml of 3% hydrogen peroxide. Thanks for contributing! I’ve changed the text.
can you send me a recipe to desinfect rooms, cars and shoes
I would stick to https://www.who.int/news-room/q-a-detail/q-a-considerations-for-the-cleaning-and-disinfection-of-environmental-surfaces-in-the-context-of-covid-19-in-non-health-care-settings.
Hello. Does 96 % ethanol kill covid19 ?
or i must dilute to 80 %?
Yes, you must dilute.
Hello
I want to make 72% disinfectant (hand rub) from 96% ethanol. How much water do i need to add to get 1000ml of 72% disinfectant? If i calculated correctly i need 616g of 96% ethanol?
Thank You 🙂
I think you need this recipe. It is WHO1 without hydrogen peroxide and glycerol, but it sticks to the 80% v/v ethanol requirement:
Substance | Mass (gram) | Volume (ml)
(Bio-)ethanol 95% v/v | 684 | 841
Glycerol | 18 | 15
Water, disinfected | 160 | 160
Totals | 934 | 1000
Personally, I hate glycerol (sticky), so I do add 175 ml water instead of 160 ml.
Thanks, but the measurements somehow don’t add up:
684+18+160 = 862g (not 934g)
and
841+15+160= 1016ml (not 1000 ml)
Am i missing something?
Thanks